B
#FoodSafety #PublicHealth #FDA

www.yahoo.com
FDA reviews potentially carcinogenic BHA chemical preservative
Add Yahoo as a preferred source to see more of our stories on Google.
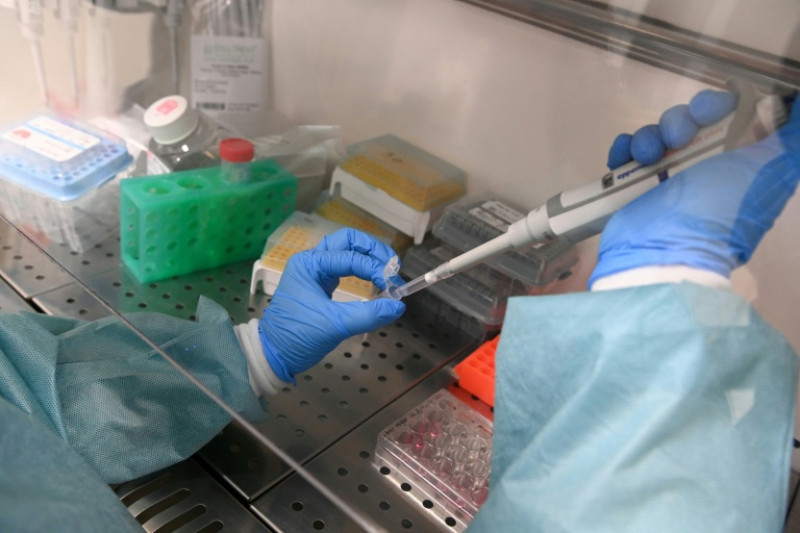
Feb. 10 (UPI) -- The Food and Drug Administration announced Tuesday it's undertaking a review of the chemical preservative butylated hydroxyanisole to determine if the potentially carcinogenic substance is safe for continued use.
#FDA #PublicHealth #Pharmaceuticals

markets.businessinsider.com
FDA intends to 'take action' against non-FDA-approved GLP-1 drugs
The U. S. Food and Drug Administration announced its intent to take decisive steps to restrict GLP-1 active pharmaceutical ingredients intended for use in non-FDA-approved compounded drugs that are being mass-marketed by companies - including Hims & Hers (HIMS) and other compounding pharmacies - as similar alternatives to FDA-approved drugs.
#EnergySupplement #FDARecall #HealthNews

nypost.com
Energy supplement recalled -- for containing an erectile dysfunction...
This energy supplement might be sweet, but the latest news about it definitely isn't.
The FDA announced this week that a Virginia company is voluntarily recalling its honey-based energy support supplement because it may contain a prescription drug commonly used to treat erectile dysfunction.
#FDA #DrugApproval #Transparency

abcnews.go.com
House lawmaker raises new concerns over FDA's ultra-fast drug review program
WASHINGTON -- A Democratic lawmaker raised new concerns about a Food and Drug Administration program designed to drastically shorten the review of certain drugs, including whether senior officials involved in the effort are complying with federal ethics rules.
#FoodSafety #PublicHealth #FDARecall

www.yahoo.com
Grocery store chain issues recall for thousands of gallons of water due to startling FDA report: 'Floating black foreign substance'
Concerns have been raised about delayed FDA warnings and potential risks to public health due to federal workforce cuts affecting food safety quality checks.